New Clinical Trials for Prostate, Kidney Cancers

Siteman Cancer Center has three new clinical trials open focused on prostate and kidney cancers. The first, a Phase II clinical trial testing a neoadjuvant immunotherapy treatment for prostate cancer, is available for men who have been diagnosed with localized prostate cancer. The study will evaluate the use of atezolizumab alone and in combination with […]
Early Phase Clinical Trial Focuses on Reducing Tumor Growth and Bone Loss in Patients with Breast Cancer and Bone Metastasis

Beginning next year, Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital will initiate a new early phase clinical trial aimed at stopping bone loss and cancer progression in patients with metastatic breast cancer (MBC). The trial is based on promising laboratory research conducted at the School of Medicine that showed a […]
Novel Lymphoma Clinical Trials Underway
In just the past five years, the development of new therapeutics has changed the paradigm for the treatment of lymphomas, thereby improving outcomes and quality of life. As research continues, a major goal is to find more tolerable treatment options with higher efficacy. At Siteman Cancer Center, 35 open clinical trials are investigating novel immunotherapies, […]
Investigating New Treatment Options – New Clinical Trial: First Personalized Vaccine for Small Cell Lung Cancer

At Siteman Cancer Center, a new clinical trial is underway that will evaluate the benefits of a neoantigen vaccine in combination with chemotherapy and immunotherapy to treat small cell lung cancer (SCLC). The rapidly growing, aggressive cancer represents about 15% of all lung cancers in the U.S. SCLC has low survival rates, with most cases […]
Washington University to test ‘homegrown’ universal CAR T-cell therapy at Siteman Cancer Center

In early 2022, immunotherapy specialists at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine will begin a new clinical trial using off-the-shelf CAR T-cell therapy for the treatment of relapsed and refractory acute lymphoblastic leukemia (ALL). The therapy, developed by Washington University researchers at Siteman, incorporates many facets of the School […]
Think Clinical Trials after First Diagnosis
Advances in cancer care are happening every day. What’s the best way to find the latest treatment options available for your patients? Check for clinical trials. While many patients think clinical trials are for those who have exhausted all current standard-of-care (SoC) treatments for their type of cancer, the best time to consider participation in […]
First-in-human clinical trials at Siteman Cancer Center to evaluate universal CAR T-cell therapies

This summer, the latest generation of CAR T-cell therapies for patients diagnosed with lymphoma, myeloma or leukemia get underway in clinical trials at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis. The cancer center was one of the first in the U.S. to offer CAR T-cell therapy for the treatment […]
Newly approved drug effective against lung cancer caused by genetic mutation

The new drug sotorasib reduces tumor size and shows promise in improving survival among patients with lung tumors caused by a specific DNA mutation, according to results of a global phase 2 clinical trial. The drug is designed to shut down the effects of the mutation, which is found in about 13% of patients with […]
Personalized cancer vaccines for breast, pancreatic cancers show promise

Researchers at Washington University School of Medicine in St. Louis have shown that personalized cancer vaccines made using DNA…
Cell-based immunotherapy shows promise against melanoma
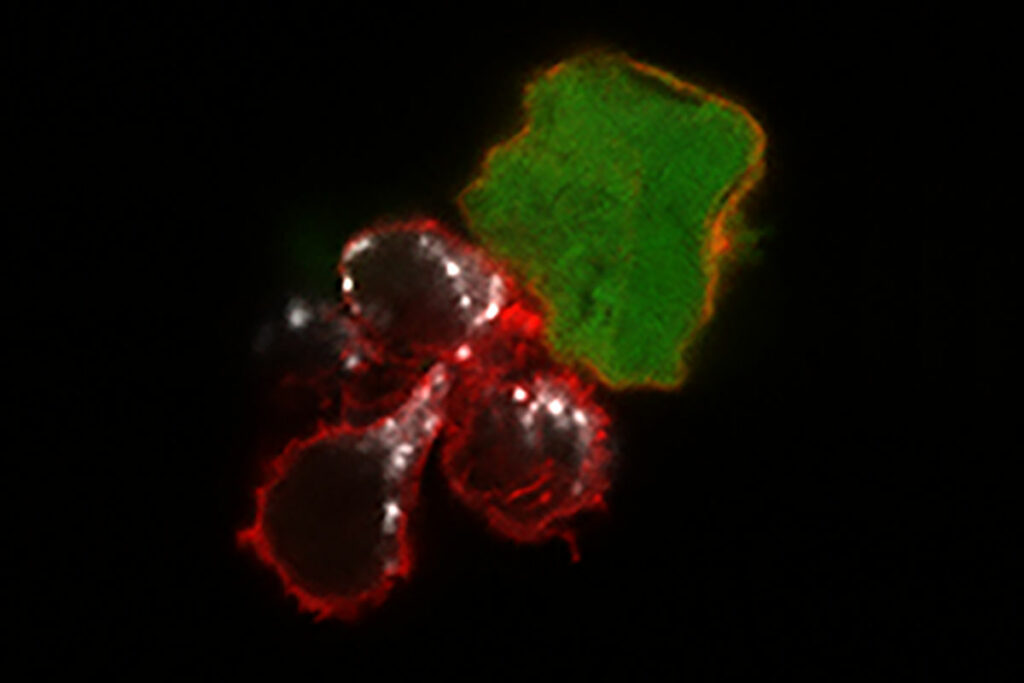
A new study from Washington University School of Medicine in St. Louis demonstrates, in mice and human cells, that a cell-based…